Table of Contents
IGCSE Chemistry: Chemical Energetics Test – ANSWERS #
How to use this answer sheet:
- Check your answers against the correct answers shown in green
- Read the explanations to understand why each answer is correct
- Review the revision notes for important concepts
- For calculations, follow the step-by-step working shown
SECTION A: MULTIPLE CHOICE ANSWERS [30 marks] #
1. Which statement correctly describes an exothermic reaction?
Answer: B – It transfers heat to the surroundings
Why this answer is correct:
Exothermic reactions release heat energy to the surroundings. This makes the surroundings warmer, not cooler. The products have less energy than reactants because energy has been given out.
Exothermic reactions release heat energy to the surroundings. This makes the surroundings warmer, not cooler. The products have less energy than reactants because energy has been given out.
2. When solid ammonium chloride dissolves in water, the temperature of the solution decreases. This process is:
Answer: D – Endothermic with positive ΔH
Why this answer is correct:
When temperature decreases, the reaction is taking heat from the surroundings (endothermic). Endothermic reactions always have positive ΔH values.
When temperature decreases, the reaction is taking heat from the surroundings (endothermic). Endothermic reactions always have positive ΔH values.
3. Which of the following is an example of an exothermic reaction?
Answer: C – Combustion of methane
Why this answer is correct:
All combustion (burning) reactions are exothermic – they release heat. Photosynthesis, thermal decomposition, and melting are all endothermic processes that need energy input.
All combustion (burning) reactions are exothermic – they release heat. Photosynthesis, thermal decomposition, and melting are all endothermic processes that need energy input.
4. The enthalpy change (ΔH) for an endothermic reaction is:
Answer: B – Always positive
Remember:
• Endothermic: ΔH is positive (+)
• Exothermic: ΔH is negative (-)
• Endothermic: ΔH is positive (+)
• Exothermic: ΔH is negative (-)
5. What is activation energy?
Answer: C – The minimum energy particles need to react when they collide
Why this answer is correct:
Activation energy (Ea) is like the “energy barrier” that must be overcome for a reaction to start. Even exothermic reactions need this initial energy input.
Activation energy (Ea) is like the “energy barrier” that must be overcome for a reaction to start. Even exothermic reactions need this initial energy input.
6. In a reaction pathway diagram, the activation energy is shown as:
Answer: B – The vertical distance from reactants to the peak of the curve
Remember: Activation energy is always measured from the starting point (reactants) up to the highest point (peak) of the reaction pathway.
7. Bond breaking is:
Answer: B – Always endothermic
Key Rule:
• Bond breaking = endothermic (needs energy)
• Bond making = exothermic (releases energy)
• Bond breaking = endothermic (needs energy)
• Bond making = exothermic (releases energy)
8. Which process releases energy?
Answer: C – Forming an H-O bond
Why this answer is correct:
Only bond formation releases energy. All the other options involve breaking bonds or separating molecules, which need energy input.
Only bond formation releases energy. All the other options involve breaking bonds or separating molecules, which need energy input.
9. Look at this reaction pathway diagram: [Reactants at 100 kJ, peak at 180 kJ, products at 60 kJ] What is the enthalpy change (ΔH) for this reaction?
Answer: A – -40 kJ/mol
ΔH = Energy of products – Energy of reactants
= 60 – 100
= -40 kJ/mol
= 60 – 100
= -40 kJ/mol
The negative value shows this is exothermic (products lower than reactants).
10. Using the same diagram from question 9, what is the activation energy?
Answer: C – 80 kJ/mol
Ea = Energy at peak – Energy of reactants
Ea = 180 – 100 = 80 kJ/mol
Ea = 180 – 100 = 80 kJ/mol
11. In the reaction: 2H₂ + O₂ → 2H₂O, how many bonds are broken in total?
Answer: B – 3
Bonds broken:
• 2 × H-H bonds (in 2H₂)
• 1 × O=O bond (in O₂)
Total = 2 + 1
= 3 bonds
• 2 × H-H bonds (in 2H₂)
• 1 × O=O bond (in O₂)
Total = 2 + 1
= 3 bonds
12. A reaction has an enthalpy change of -286 kJ/mol. This means:
Answer: B – 286 kJ of energy is released to the surroundings
Remember: Negative ΔH means exothermic (energy released). The value tells us how much energy per mole.
13. Which factor does NOT affect the enthalpy change of a reaction?
Answer: C – The presence of a catalyst
Important: Catalysts lower activation energy but do NOT change the enthalpy change (ΔH). The energy difference between reactants and products stays the same.
14. When calculating enthalpy change using bond energies, the formula is:
Answer: B – ΔH = Energy of bonds broken – Energy of bonds formed
Remember this formula:
ΔH = Energy IN (to break bonds) – Energy OUT (when bonds form)
ΔH = Energy IN (to break bonds) – Energy OUT (when bonds form)
15. A student touches a test tube where magnesium is reacting with hydrochloric acid. The test tube feels hot. This indicates:
Answer: D – An exothermic reaction with negative ΔH
Hot test tube = heat released = exothermic = negative ΔH
16. Which statement about catalysts is correct?
Answer: B – They decrease the activation energy
Catalysts provide an alternative pathway with lower activation energy, making reactions happen more easily.
17. In the reaction N₂ + 3H₂ → 2NH₃, how many N-H bonds are formed?
Answer: D – 6
Calculation:
• Each NH₃ molecule has 3 N-H bonds
• 2 NH₃ molecules are formed
• Total = 2 × 3 = 6 N-H bonds
• Each NH₃ molecule has 3 N-H bonds
• 2 NH₃ molecules are formed
• Total = 2 × 3 = 6 N-H bonds
18. The bond energy of H-H is 435 kJ/mol. This means:
Answer: B – 435 kJ is needed to break one mole of H-H bonds
Bond energy is the energy needed to break bonds (endothermic process).
19. Which reaction pathway diagram represents an endothermic reaction?
Answer: B – Diagram showing products higher than reactants
Endothermic: products have more energy than reactants (uphill reaction).
20. During respiration, glucose reacts with oxygen to produce carbon dioxide and water. This process:
Answer: B – Releases heat to keep the body warm
Respiration is exothermic – it releases energy that our body uses for warmth and other processes.
21. A reaction has these bond energies: Bonds broken: 1000 kJ, Bonds formed: 1200 kJ What is the enthalpy change?
Answer: B – -200 kJ/mol
ΔH = Bonds broken – Bonds formed
= 1000 – 1200
= -200 kJ/mol
= 1000 – 1200
= -200 kJ/mol
22. Why does a match need to be struck before it burns?
Answer: B – To provide the activation energy
Even exothermic reactions need activation energy to start. Striking provides this initial energy.
23. Which diagram correctly shows the effect of a catalyst?
Answer: A – Lower peak, same start and end points
Catalysts lower the activation energy (peak) but don’t change reactant or product energy levels.
24. The reaction 2HI → H₂ + I₂ involves breaking 2 H-I bonds. If the H-I bond energy is 298 kJ/mol, how much energy is needed to break all bonds?
Answer: C – 596 kJ
Energy needed = 2 × 298
= 596 kJ
= 596 kJ
25. Which process is endothermic?
Answer: C – Thermal decomposition of copper carbonate
Thermal decomposition reactions need continuous heat input to break down compounds.
26. In an experiment, citric acid reacts with sodium hydrogencarbonate. The temperature drops from 20°C to 15°C. What type of reaction is this?
Answer: B – Endothermic with energy absorbed
Temperature drop = heat absorbed from surroundings = endothermic
27. The reaction CH₄ + 2O₂ → CO₂ + 2H₂O has ΔH = -890 kJ/mol. This means:
Answer: B – 890 kJ is released when one mole of methane burns
Negative ΔH = exothermic = energy released
28. Which bond is likely to have the highest bond energy?
Answer: C – C≡C triple bond
More bonds between atoms = stronger = higher bond energy. Triple > Double > Single
29. A reaction profile shows the peak at 150 kJ, reactants at 50 kJ, and products at 30 kJ. Which statement is correct?
Answer: B – ΔH = -20 kJ, Ea = 100 kJ
ΔH = Products – Reactants
= 30 – 50
= -20 kJ
Ea = Peak – Reactants
= 150 – 50
= 100 kJ
= 30 – 50
= -20 kJ
Ea = Peak – Reactants
= 150 – 50
= 100 kJ
30. Why must all chemical reactions have an activation energy?
Answer: B – To prevent all reactions happening instantly
Activation energy acts as an energy barrier. Without it, all possible reactions would happen immediately, which would be chaotic!
SECTION B: WRITTEN RESPONSE ANSWERS [70 marks] #
1. Define the following terms: [4]
- Exothermic reaction (2 marks)
- Activation energy (2 marks)
a) Exothermic reaction:
An exothermic reaction is a chemical reaction that transfers thermal energy (heat) to the surroundings, causing the temperature of the surroundings to increase.
Mark scheme: 1 mark for mentioning energy transfer/release, 1 mark for mentioning surroundings/temperature increase
b) Activation energy:Activation energy (Ea) is the minimum energy that colliding particles must have for a reaction to occur when they collide.
Mark scheme: 1 mark for “minimum energy”, 1 mark for “particles must have to react/collide successfully”
2. Give two examples of exothermic reactions and two examples of endothermic reactions that happen in everyday life. [4]
Exothermic reactions:
1. Combustion/burning of fuels (e.g., wood, gas, candle)
2. Respiration in living organisms
Also accept: neutralization reactions, rusting of iron, hand warmers
Endothermic reactions:
1. Photosynthesis in plants
2. Melting of ice / evaporation of water
Also accept: dissolving ammonium chloride, thermal decomposition, cold packs
1. Combustion/burning of fuels (e.g., wood, gas, candle)
2. Respiration in living organisms
Also accept: neutralization reactions, rusting of iron, hand warmers
Endothermic reactions:
1. Photosynthesis in plants
2. Melting of ice / evaporation of water
Also accept: dissolving ammonium chloride, thermal decomposition, cold packs
Mark scheme: 1 mark for each correct example (4 × 1 mark)
3. A student adds calcium oxide to water in a beaker. The reaction can be shown as: CaO + H₂O → Ca(OH)₂
- The student notices the beaker becomes very hot. What type of reaction is this? [1]
- What is the sign of ΔH for this reaction? Explain your answer. [2]
- Sketch a reaction pathway diagram for this reaction. Label the reactants, products, and enthalpy change. [3]
a)
Exothermic reaction
b)
ΔH is negative. Because the reaction releases heat to the surroundings (making the beaker hot), it is exothermic, and all exothermic reactions have negative ΔH values.
Mark scheme: 1 mark for “negative”, 1 mark for correct explanatio
4. Explain why bond breaking is endothermic and bond making is exothermic. Use the idea of energy in your answer. [4]
Bond breaking is endothermic because energy must be supplied to overcome the attractive forces between atoms in a bond. This is like pulling apart two magnets – you need to put energy in to separate them.
Bond making is exothermic because when atoms come together to form bonds, they release energy as they reach a more stable state. This is like magnets snapping together – they release energy when they connect.
Bond making is exothermic because when atoms come together to form bonds, they release energy as they reach a more stable state. This is like magnets snapping together – they release energy when they connect.
Mark scheme:
• 2 marks for bond breaking explanation (1 mark for endothermic, 1 mark for energy needed/forces)
• 2 marks for bond making explanation (1 mark for exothermic, 1 mark for energy released/stability)
• 2 marks for bond breaking explanation (1 mark for endothermic, 1 mark for energy needed/forces)
• 2 marks for bond making explanation (1 mark for exothermic, 1 mark for energy released/stability)
5. The diagram shows a reaction pathway for the decomposition of hydrogen peroxide: [2H₂O₂ → 2H₂O + O₂ with reactants at 0 kJ, peak at 75 kJ, products at -196 kJ]
- What is the activation energy for this reaction? [1]
- What is the enthalpy change (ΔH) for this reaction? [1]
- Is this reaction exothermic or endothermic? Explain how you know. [2]
a)
Ea = 75 kJ
Ea = peak energy – reactant energy
= 75 – 0
= 75 kJ
b)
= 75 – 0
= 75 kJ
ΔH = -196 kJ
ΔH = product energy – reactant energy
= -196 – 0
= -196 kJ
c)
= -196 – 0
= -196 kJ
Exothermic. The products have lower energy than the reactants (products at -196 kJ, reactants at 0 kJ), and ΔH is negative.
Mark scheme: 1 mark for lower peak, 1 mark for same start and end points
6. Photosynthesis is an important endothermic reaction in plants: 6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
- Where does the energy for this reaction come from? [1]
- Explain why this reaction is endothermic in terms of the energy of reactants and products. [2]
- What would be the sign of ΔH for this reaction? [1]
a)
Sunlight / light energy / the Sun
b)
The reaction is endothermic because the products (glucose and oxygen) have more energy than the reactants (carbon dioxide and water). Energy must be absorbed from the surroundings to increase the energy level.
c)
Positive (+)
Remember: Endothermic reactions always have positive ΔH
7. Draw and label a complete reaction pathway diagram for an exothermic reaction where: [4]
- The reactants have an energy of 150 kJ/mol
- The products have an energy of 50 kJ/mol
- The activation energy is 80 kJ/mol
Key points for the diagram:
• Reactants at 150 kJ
• Peak at 230 kJ (150 + 80)
• Products at 50 kJ
• Ea arrow from 150 to 230 (80 kJ)
• ΔH arrow from 150 to 50 (-100 kJ)
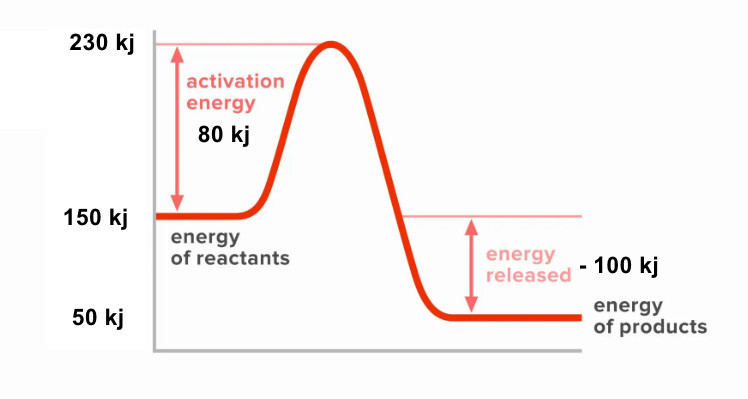
• Reactants at 150 kJ
• Peak at 230 kJ (150 + 80)
• Products at 50 kJ
• Ea arrow from 150 to 230 (80 kJ)
• ΔH arrow from 150 to 50 (-100 kJ)

8. Calculate the enthalpy change for the reaction: H₂ + Cl₂ → 2HCl [5]
Bond energies: H-H = 435 kJ/mol, Cl-Cl = 240 kJ/mol, H-Cl = 430 kJ/mol
Bond energies: H-H = 435 kJ/mol, Cl-Cl = 240 kJ/mol, H-Cl = 430 kJ/mol
Step 1: Identify bonds broken
• 1 × H-H bond = 1 × 435 = 435 kJ
• 1 × Cl-Cl bond = 1 × 240 = 240 kJ
Total energy to break bonds = 435 + 240 = 675 kJ
Step 2: Identify bonds formed
• 2 × H-Cl bonds = 2 × 430 = 860 kJ
Total energy released = 860 kJ
Step 3: Calculate ΔH
ΔH = Energy to break bonds – Energy released when bonds form
ΔH = 675 – 860
= -185 kJ/mol
• 1 × H-H bond = 1 × 435 = 435 kJ
• 1 × Cl-Cl bond = 1 × 240 = 240 kJ
Total energy to break bonds = 435 + 240 = 675 kJ
Step 2: Identify bonds formed
• 2 × H-Cl bonds = 2 × 430 = 860 kJ
Total energy released = 860 kJ
Step 3: Calculate ΔH
ΔH = Energy to break bonds – Energy released when bonds form
ΔH = 675 – 860
= -185 kJ/mol
Answer: ΔH = -185 kJ/mol (exothermic)
Mark scheme: 1 mark for bonds broken, 1 mark for total (675), 1 mark for bonds formed, 1 mark for total (860), 1 mark for correct ΔH
9. A student investigates the reaction between zinc and copper sulfate solution: Zn + CuSO₄ → ZnSO₄ + Cu The temperature rises from 18°C to 26°C.
- Is this reaction exothermic or endothermic? [1]
- Explain your answer to part (a) using the temperature change. [2]
- Predict the sign of ΔH for this reaction. [1]
a)
Exothermic
b)
The temperature increased by 8°C (from 18°C to 26°C). This shows that heat energy was released to the surroundings, which is characteristic of an exothermic reaction.
c)
Negative (-)
10. The combustion of ethanol: C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O
- State whether this reaction is exothermic or endothermic. [1]
- Explain why all combustion reactions have the same type of energy change. [2]
- Why does ethanol need a spark or flame to start burning? [2]
a)
Exothermic
b)
All combustion reactions are exothermic because they involve reacting with oxygen to form stable products (like CO₂ and H₂O). The bonds in the products are stronger than in the reactants, so energy is released.
c)
The spark or flame provides the activation energy needed to start the reaction. Even though combustion releases energy overall, particles still need this initial energy to break the first bonds and begin reacting.
11. Calculate the enthalpy change for: CH₄ + 2O₂ → CO₂ + 2H₂O [6]
Bond energies: C-H = 410 kJ/mol, O=O = 498 kJ/mol, C=O = 740 kJ/mol, O-H = 460 kJ/mol
Bond energies: C-H = 410 kJ/mol, O=O = 498 kJ/mol, C=O = 740 kJ/mol, O-H = 460 kJ/mol
Start by drawing the structure so that you can count bonds
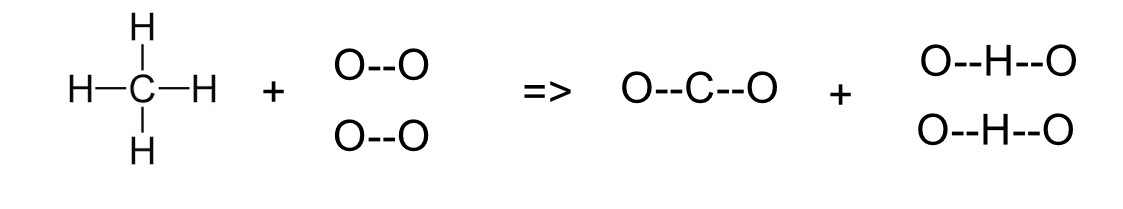
Step 1: Count bonds broken
CH₄ has 4 C-H bonds
2O₂ has 2 O=O bonds
• 4 × C-H = 4 × 410 = 1640 kJ
• 2 × O=O = 2 × 498 = 996 kJ
Total energy to break bonds = 1640 + 996 = 2636 kJ
————————————————
Step 2: Count bonds formed
CO₂ has 2 C=O bonds
2H₂O has 4 O-H bonds (2 molecules × 2 bonds each)
• 2 × C=O
= 2 × 740
= 1480 kJ
• 4 × O-H
= 4 × 460
= 1840 kJ
Total energy released = 1480 + 1840
= 3320 kJ
————————————————
Step 3: Calculate ΔH
ΔH = 2636 – 3320
= -684 kJ/mol
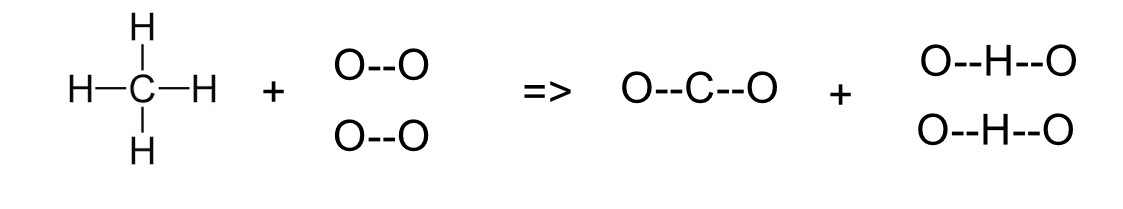
Step 1: Count bonds broken
CH₄ has 4 C-H bonds
2O₂ has 2 O=O bonds
• 4 × C-H = 4 × 410 = 1640 kJ
• 2 × O=O = 2 × 498 = 996 kJ
Total energy to break bonds = 1640 + 996 = 2636 kJ
————————————————
Step 2: Count bonds formed
CO₂ has 2 C=O bonds
2H₂O has 4 O-H bonds (2 molecules × 2 bonds each)
• 2 × C=O
= 2 × 740
= 1480 kJ
• 4 × O-H
= 4 × 460
= 1840 kJ
Total energy released = 1480 + 1840
= 3320 kJ
————————————————
Step 3: Calculate ΔH
ΔH = 2636 – 3320
= -684 kJ/mol
Answer: ΔH = -684 kJ/mol
Mark scheme: 1 mark for each correct bond count (4 marks), 1 mark for correct totals, 1 mark for correct ΔH
12. A reaction has the following energy values: Energy needed to break bonds = 850 kJ/mol, Energy released when bonds form = 920 kJ/mol
- Calculate the enthalpy change (ΔH) for this reaction. [2]
- Is the reaction exothermic or endothermic? [1]
- Sketch a reaction pathway diagram for this reaction. [3]
a)
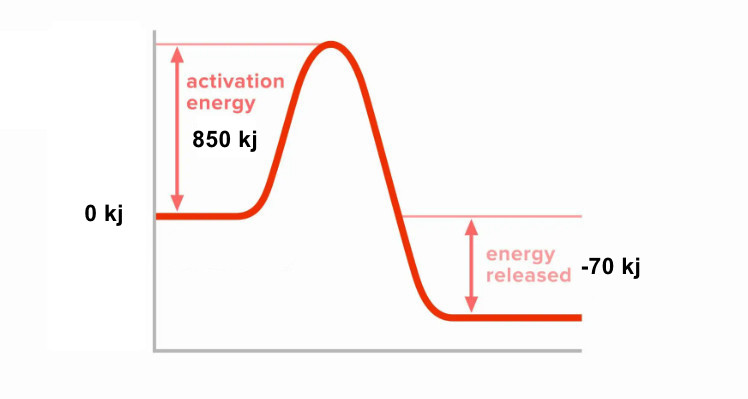
ΔH = Energy to break bonds – Energy released
ΔH = 850 – 920
= -70 kJ/mol
b)
ΔH = 850 – 920
= -70 kJ/mol
Exothermic (because ΔH is negative)
c)

13. Explain the difference between enthalpy change (ΔH) and activation energy (Ea). [4]
Enthalpy change (ΔH): This is the overall energy difference between products and reactants. It tells us if a reaction is exothermic (negative ΔH) or endothermic (positive ΔH). It measures the net energy released or absorbed.
Activation energy (Ea): This is the minimum energy needed to start a reaction. It’s the energy barrier that must be overcome for particles to react successfully when they collide. All reactions need activation energy, even exothermic ones.
Activation energy (Ea): This is the minimum energy needed to start a reaction. It’s the energy barrier that must be overcome for particles to react successfully when they collide. All reactions need activation energy, even exothermic ones.
Mark scheme: 2 marks for ΔH explanation, 2 marks for Ea explanation
Final Revision Tips:
• Always check the sign of ΔH: negative = exothermic, positive = endothermic
• Remember: Breaking bonds needs energy, making bonds releases energy
• Draw bond structures when calculating ΔH to avoid missing bonds
• Activation energy is ALWAYS positive (energy needed)
• Temperature rise = exothermic, temperature fall = endothermic
• Always check the sign of ΔH: negative = exothermic, positive = endothermic
• Remember: Breaking bonds needs energy, making bonds releases energy
• Draw bond structures when calculating ΔH to avoid missing bonds
• Activation energy is ALWAYS positive (energy needed)
• Temperature rise = exothermic, temperature fall = endothermic




