- 5.1 Exothermic and Endothermic Reactions
- Enthalpy Change and Activation Energy
- What is Enthalpy Change (ΔH)?
- What is Activation Energy (Ea)?
- Exothermic Reactions
- Endothermic Reactions
- Reaction Pathway Diagrams
- Supplement: Enthalpy Change (ΔH)
- Activation Energy (Ea)
- Drawing and Labeling Reaction Pathway Diagrams
- Bond Breaking and Bond Making
- Calculating Enthalpy Change Using Bond Energies
- Summary of Key Points
5.1 Exothermic and Endothermic Reactions #
IGCSE Chemistry Topic 5 – Chemical Energetics
Think of it like this – to take molecules apart (break bonds), you need to put energy in, like pulling apart two magnets.
When molecules come together (make bonds), they release energy, like when magnets snap together.
The overall energy change tells us if a reaction is exothermic or endothermic.
Enthalpy Change and Activation Energy #
What is Enthalpy Change (ΔH)? #
ΔH is negative (-) → Exothermic reaction (gives out heat)
ΔH is positive (+) → Endothermic reaction (takes in heat)
• Burning methane: ΔH = -890 kJ/mol (exothermic – releases 890 kJ of heat per mole)
• Photosynthesis: ΔH = +2802 kJ/mol (endothermic – absorbs 2802 kJ of heat per mole)
What is Activation Energy (Ea)? #
- ALL reactions need activation energy to start (even exothermic ones)
- Activation energy is always positive (energy must be added)
- Higher activation energy = reaction is harder to start
- Lower activation energy = reaction starts more easily
- Catalysts work by lowering the activation energy
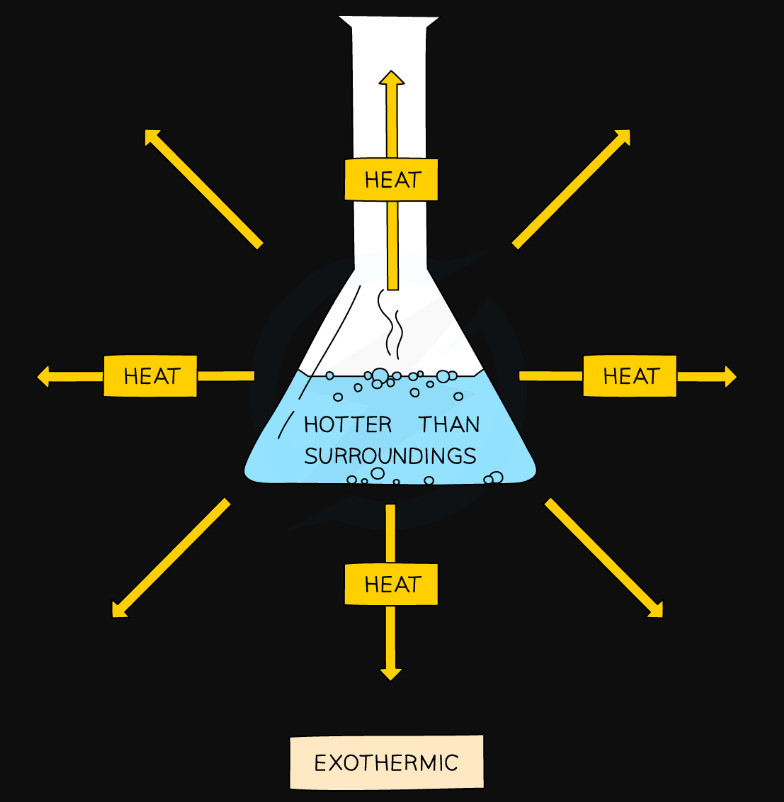
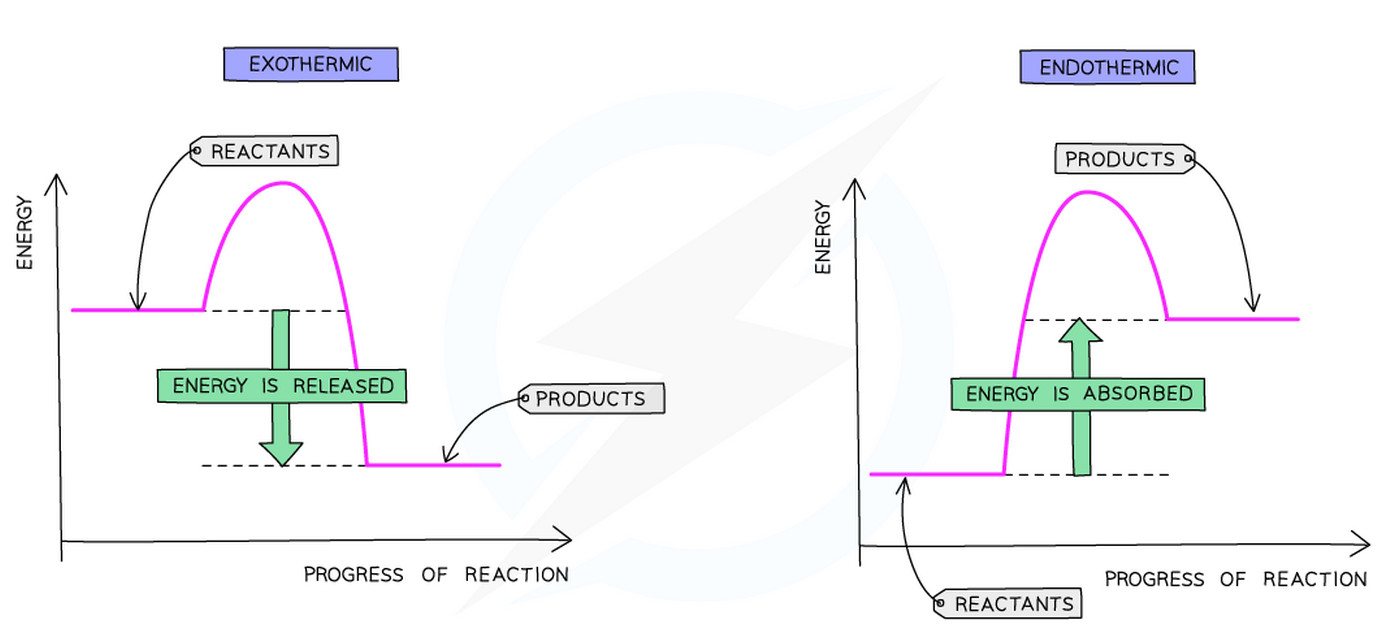
Exothermic Reactions #
- Temperature of surroundings increases
- Heat energy is released to the surroundings
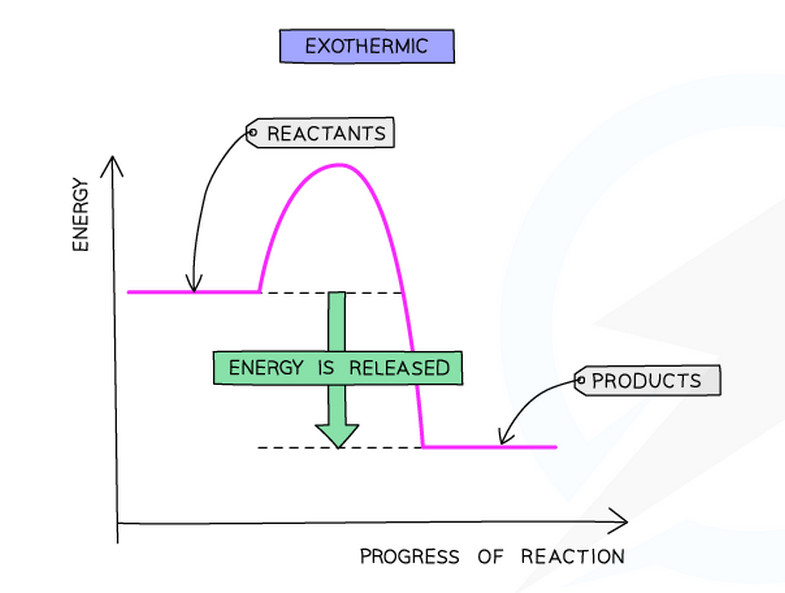
- Products have less energy than reactants
- More energy is released when making new bonds than is needed to break the old bonds
- The enthalpy change (ΔH) is -negative
Endothermic Reactions #
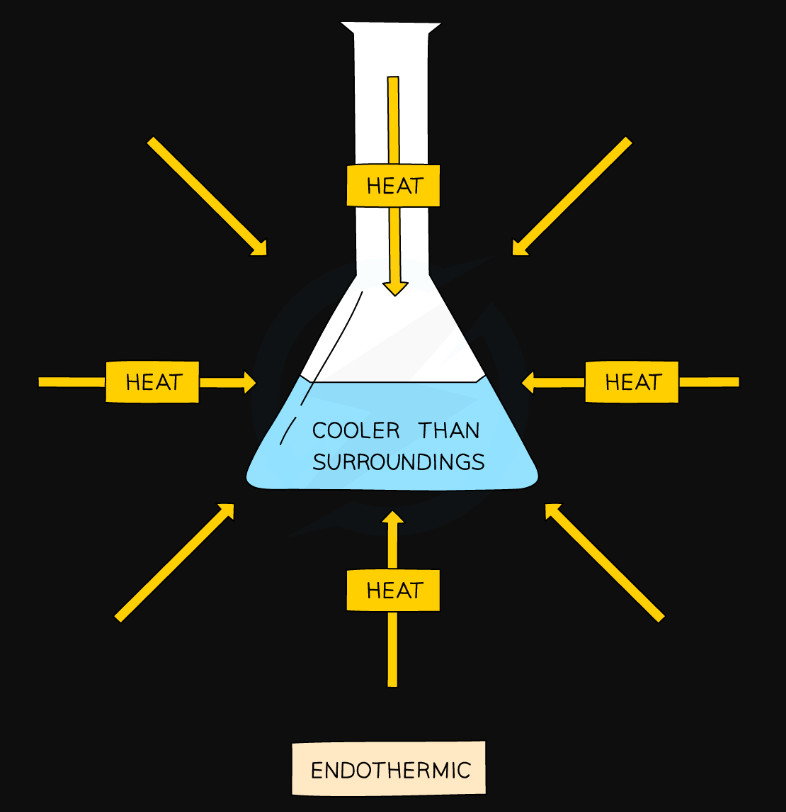
- Temperature of surroundings decreases
- Heat energy is absorbed from the surroundings
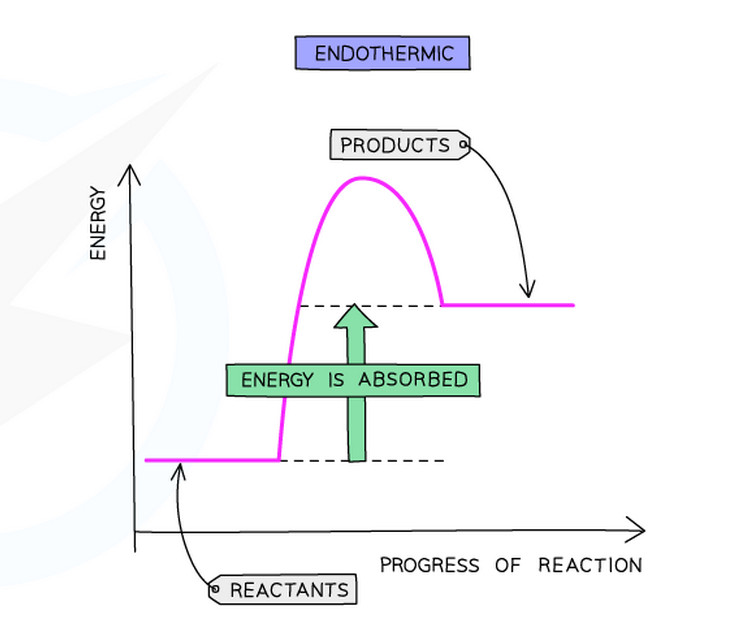
- Products have more energy than reactants
- More energy is needed to break the old bonds than is released when making new bonds
- The enthalpy change (ΔH) is +positive
Reaction Pathway Diagrams #
Supplement: Enthalpy Change (ΔH) #
Exothermic reactions: ΔH is negative (e.g., ΔH = -286 kJ/mol)
Endothermic reactions: ΔH is positive (e.g., ΔH = +178 kJ/mol)
Activation Energy (Ea) #
- It’s always positive (energy must be added)
- Lower activation energy means reactions happen more easily
- Catalysts work by lowering the activation energy
- On energy diagrams, it’s the height from reactants to the peak
Drawing and Labeling Reaction Pathway Diagrams #
- Draw axes: Vertical axis = Energy, Horizontal axis = Progress of reaction
- Mark reactants: Draw a horizontal line on the left showing the energy level of reactants
- Draw the curve: Draw a sqaure curve going up (activation energy) across and then down to products
- Mark products: Draw a horizontal line on the right showing the energy level of products
- Add arrow for ΔH: Draw a vertical arrow from reactants to products level
- Add arrow for Ea: Draw a vertical arrow from reactants to the peak of the curve
- Label everything: Label reactants, products, ΔH, and Ea
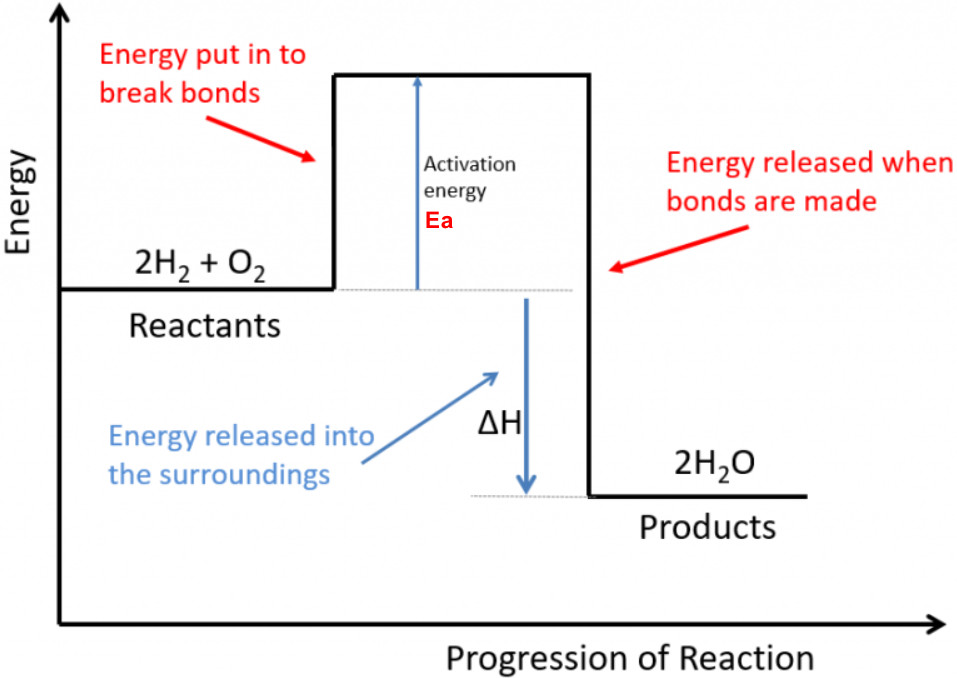
Bond Breaking and Bond Making #
2. Bond making is ALWAYS Exothermic (releases energy)
$\Delta H = \text{Energy to break bonds} – \text{Energy released when bonds form}$
Calculating Enthalpy Change Using Bond Energies #
Worked Example: Formation of Ammonia #
Reaction: N₂ + 3H₂ → 2NH₃
Given bond energies:
| Bond | N≡N | H—H | N—H |
|---|---|---|---|
| Bond energy (kJ/mol) | 945 | 435 | 390 |
Step 1: Count and calculate energy to break bonds
Bonds broken in reactants:
• 1 × N≡N bond = 1 × 945 = 945 kJ
• 3 × H—H bonds = 3 × 435 = 1305 kJ
Total energy to break bonds = 945 + 1305 = 2250 kJ
Step 2: Count and calculate energy released when bonds form
Bonds formed in products:
• 6 × N—H bonds
• 6 × 390 = 2340 kJ
Total energy released = 2340 kJ
Step 3: Calculate ΔH
$\Delta H = 2250 – 2340 = -90 \text{ kJ/mol}$
Answer: ΔH = -90 kJ/mol (negative, so the reaction is exothermic)
Example 2: Decomposition of Water #
Reaction: 2H₂O → 2H₂ + O₂
Given bond energies:
| Bond | O—H | H—H | O=O |
|---|---|---|---|
| Bond energy (kJ/mol) | 464 | 435 | 498 |
Step 1: Count and calculate energy to break bonds
Bonds broken in reactants:
• 4 × O—H bonds
• 4 × 464 = 1856 kJ
Total energy to break bonds = 1856 kJ
Step 2: Count and calculate energy released when bonds form
Bonds formed in products:
• 2 × H—H bonds = 2 × 435 = 870 kJ
• 1 × O=O bond = 1 × 498 = 498 kJ
Total energy released = 870 + 498 = 1368 kJ
Step 3: Calculate ΔH
$\Delta H = 1856 – 1368 = +488 \text{ kJ/mol}$
Answer: ΔH = +488 kJ/mol (positive, so the reaction is endothermic)
- Always draw out the structural formulas with all bonds shown – this makes counting much easier
- Count bonds carefully – remember that N₂ has a triple bond (N≡N) and O₂ has a double bond (O=O)
- Don’t forget to multiply by the number of molecules (use the coefficients in the equation)
- Check your sign: negative ΔH = exothermic, positive ΔH = endothermic
Summary of Key Points #
- Exothermic reactions: Release heat to surroundings, temperature increases, ΔH is negative
- Endothermic reactions: Absorb heat from surroundings, temperature decreases, ΔH is positive
- Activation energy (Ea): Minimum energy needed for particles to react when they collide
- Bond breaking: Always endothermic (needs energy)
- Bond making: Always exothermic (releases energy)
- Calculating ΔH: Energy to break bonds minus energy released when bonds form
- Reaction pathway diagrams: Must show:
- reactants
- products
- ΔH
- Activation Energy – Ea




