Matter is anything that has mass and takes up space. Everything around us is made of matter – from the air we breathe to the water we drink and the chair we sit on. Matter exists in three main states: solid, liquid, and gas. The differences between these states can be explained using the particle model of matter.
1. The Particle Model of Matter #
The particle model helps us understand how matter behaves. According to this model:
- All matter is made up of tiny particles (atoms or molecules)
- These particles are always in motion
- There are forces of attraction between particles
- The arrangement and movement of these particles determine the state of matter
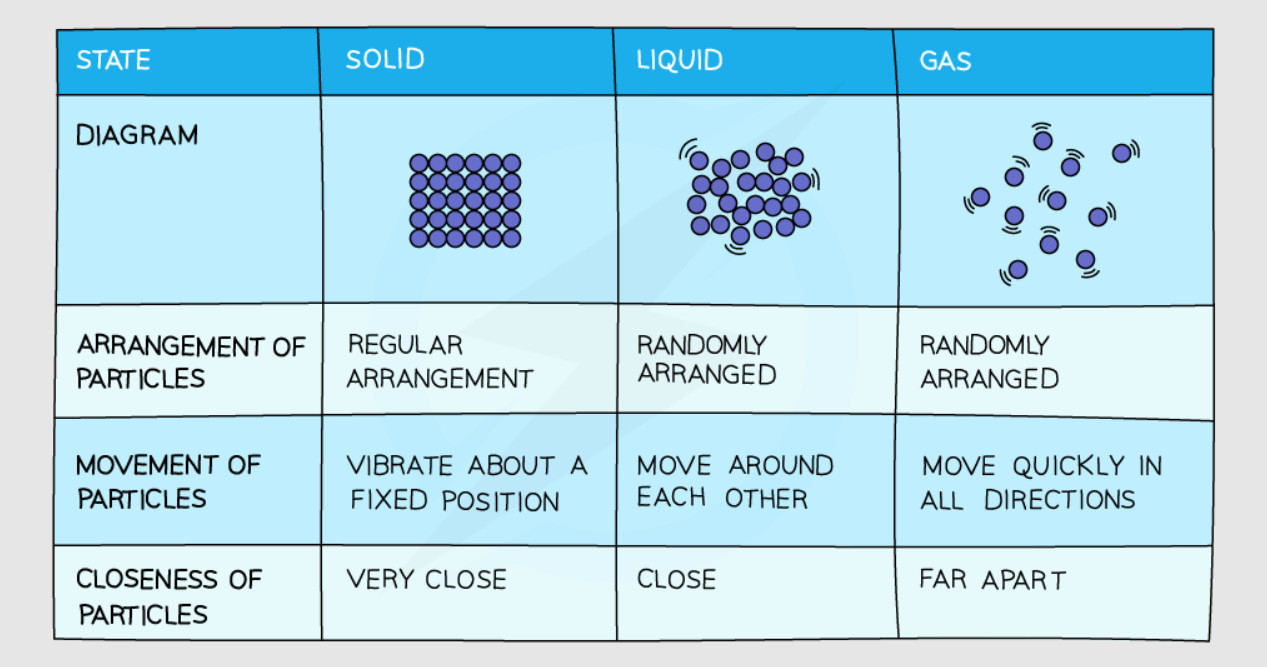
2. Solids #
Solids have a fixed shape and volume. They cannot flow and are difficult to compress.
Particle Arrangement in Solids #
- Spacing: Particles are packed very closely together in a regular pattern
- Forces: Strong attractive forces hold particles in fixed positions
- Movement: Particles can only vibrate about their fixed positions
- Energy: Particles have the least amount of energy compared to other states
Properties of Solids #
- Cannot flow (rigid)
- Have a fixed shape
- Cannot be compressed easily
- Do not diffuse into one another easily
- Example: ice, wood, metals
3. Liquids #
Liquids have a fixed volume but can change shape to fill the bottom of their container. They can flow and are difficult to compress.
Particle Arrangement in Liquids #
- Spacing: Particles are close together but not in fixed positions
- Forces: Moderate attractive forces between particles
- Movement: Particles can move past each other (flow)
- Energy: Particles have more energy than in solids
Properties of Liquids #
- Can flow to take the shape of the container (not rigid)
- Have a fixed volume
- Cannot be compressed easily
- Can diffuse into one another
- Example: water, oil, milk
4. Gases #
Gases have no fixed shape or volume. They expand to fill their entire container, can flow easily, and are easily compressed.
Particle Arrangement in Gases #
- Spacing: Particles are very far apart from each other
- Forces: Very weak attractive forces between particles
- Movement: Particles move quickly and randomly in all directions
- Energy: Particles have the most energy compared to other states
Properties of Gases #
- Flow easily
- No fixed shape (take the shape of the entire container)
- No fixed volume (fill the entire container)
- Can be compressed easily
- Diffuse very quickly
- Example: oxygen, carbon dioxide, steam
5. Comparing the Three States of Matter #
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape | Fixed | Takes shape of container (bottom) | Takes shape of entire container |
| Volume | Fixed | Fixed | Fills the container |
| Particle arrangement | Regular pattern | Random but close | Random and far apart |
| Particle movement | Vibration only | Can move past each other | Fast, random movement |
| Forces between particles | Strong | Moderate | Very weak |
| Compressibility | Very difficult | Difficult | Easy |
| Ability to flow | Cannot flow | Can flow | Flows easily |
| Diffusion | Very slow | Slow | Fast |
6. Changes of State #
Matter can change from one state to another when energy is added or removed. These changes are physical changes (not chemical) because the substance remains the same.
Types of State Changes #
- Melting: Solid → Liquid (energy added)
- Freezing: Liquid → Solid (energy removed)
- Vaporization/Evaporation: Liquid → Gas (energy added)
- Condensation: Gas → Liquid (energy removed)
- Sublimation: Solid → Gas (energy added)
- Deposition: Gas → Solid (energy removed)
What Happens to Particles During State Changes? #
During a change of state:
- Temperature remains constant even though energy is being added or removed
- The energy added or removed changes the arrangement and movement of particles
- For example, during melting, added energy breaks some of the bonds between particles in a solid, allowing them to move more freely as a liquid
7. Particle Velocity and Distribution #
Not all particles in a substance move at the same speed. There is a distribution of velocities.
Velocity Distribution #
- In gases, some particles move very slowly while others move very quickly
- The average velocity increases as temperature increases
- In solids, particles vibrate more vigorously as temperature increases
- In liquids, particles move faster as temperature increases




